

Julien Mawuena - Customer Success
For sanitary and compliance reasons, traceability systems are particularly sensitive in certain industries: pharmaceutical, dairy or other food industries. European regulations require that each batch of products put on the market be linked to a traceability report. This often tedious operation can be totally automated and simplified.
In the event of an alert concerning defective batches, manufacturers must analyze the entire production chain, in order to be able to immediately trace the incriminated stocks. It is the company’s responsibility to identify the causes of the incidents and to remedy as quickly as possible the points where quality is lacking. The implementation of an internal traceability of production operations is essential, considering that the recall and destruction of goods can have a serious impact on the image of a brand in Europe or in the world.
If, for buyers and consumers, the constraints and rules of traceability are materialized by a simple label affixed to each product, food traceability softwares at the scale of a production site are often complex. They require the cross-referencing of heterogeneous data from different sources, in order to trace all the production stages to the finished product.
This data is collected in several ways. On the one hand, information related to the types of batches produced and to quality is provided by the company’s software such as ERP and LIMS. On the other hand, production data are generated by the supervision and the machines’ PLC. Some of these data can also be manually transferred to paper. Their global processing is costly in time spent for each production unit. Thus, the production of a traceability report can take up to a few hours and mobilize several people, responsible for the various sources of data.
The development of a global traceability system connected to all departments of the plant can improve two essential points. On the one hand, at the operational level, data collection processes are more fluid and reliable. On the other hand, in the event of an incident, the production unit is much more responsive: it can quickly track and isolate the faulty factor, at the crossroads of a series of flows. For example, in the case of a contaminated batch of meat, it is possible to quickly trace all of the processing steps that caused the incident and, if necessary, to identify the origin of the product right back to the supplier.
It is clear that the information systems of production sites often remain separated, especially in the food industry. Despite the cooperation between the different departments of the factory, the collection and transmission of information can be long and complex. The objective to improve the traceability process is first to create a digital continuity of the entire production process.
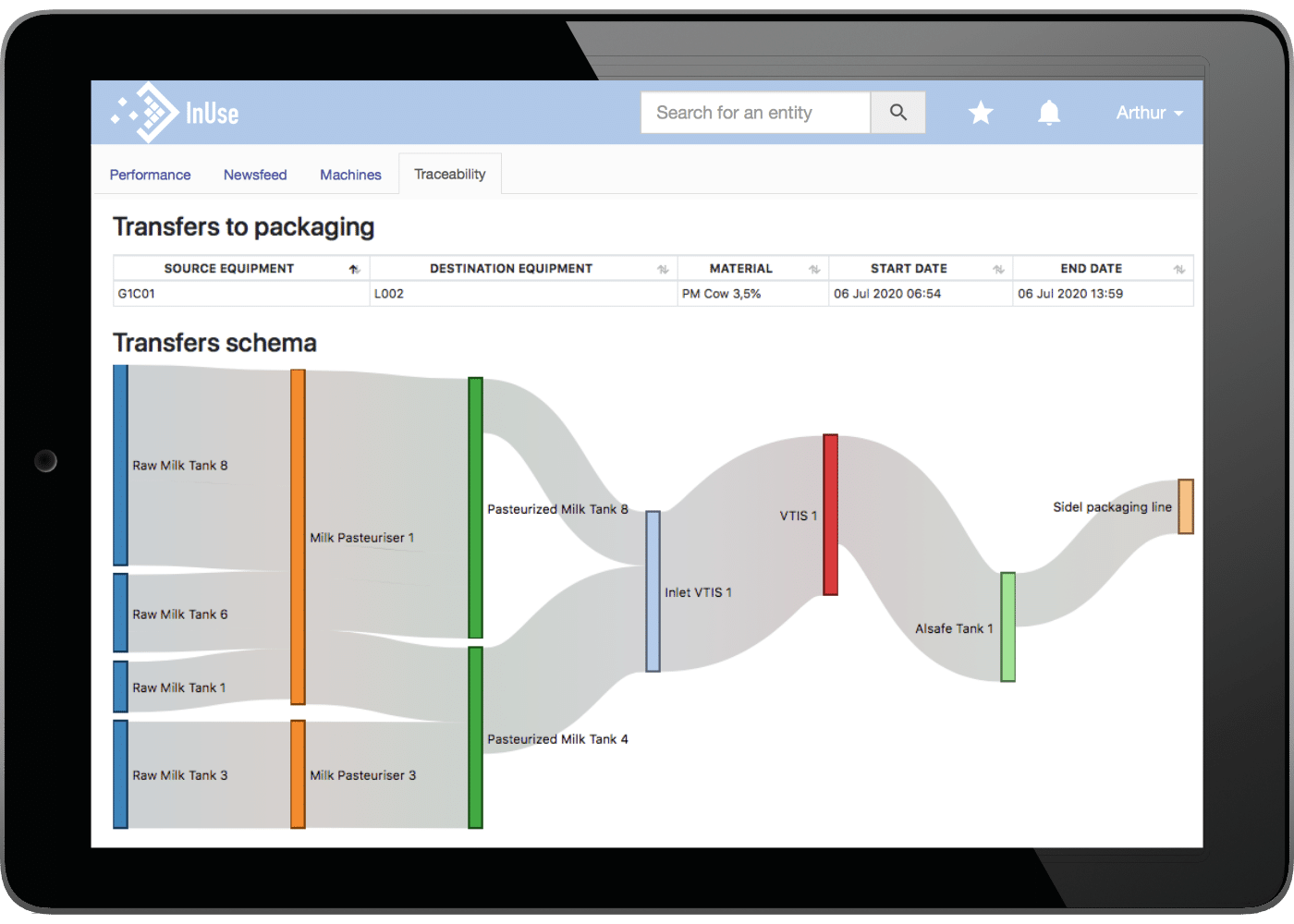
The traceability module developed by InUse enables the seamless integration and collection of heterogeneous data in the cloud that is usually scattered throughout the factory: information from the ERP, production data collected at the machine level, quality test results. This digital continuity is also created horizontally between the different departments of the production chain, such as the reception of raw materials, the process, packaging and packing.
The challenge is to re-consolidate this data solely on the basis of its time stamp and the description of the business processes in force in the factory. This method has the advantage of being much more flexible and faster than having to model all the processes in the plant.
The reconstitution of all the production events through data allows to precisely describe the life cycle of a production batch: type and quantity of raw materials delivered by the different operators, ingredients used in the recipe, production data (batch number, quantity produced, date and time…), quality test results.
Accessible from any device (smartphone, tablet, computer) these traceability reports also include a key functionality presenting all material transfers during production in the form of ergonomic diagrams.
Moreover, if the CIP system is automated and connected, it is then possible to complete the reports with cleaning cycles data such as the status of the cleaning (compliant or not) and their timestamp in order to reinforce the sanitary control of each batch. The use of this cleaning data is useful for factories to assess whether production has been carried out in accordance with the required hygiene rules, to anticipate sanitary problems and to reinforce controls.
For quality departments, the implementation of a food traceability software can reduce the time spent producing and checking traceability reports by up to 90%. As an example, the Hellenic Dairies Group (European leader in the dairy products market) has deployed the InUse traceability module in one of its production plants (see video). Based on 50 connected equipments, it is now possible to continuously generate traceability reports with a single click via the application. Previously, this type of report required an average of 2 hours of team time.
In addition, the InUse solution gives the quality department an overview of the completeness of traceability reports. Missing information can be identified immediately, as opposed to a situation where information is collected manually and incrementally. Furthermore, in case of an alert or an audit required by law and health regulations, the connected solution guarantees optimal reactivity.
Finally, automated data collection significantly strengthens the reliability and integrity of reports. This process favors the dematerialization of operations in the plant and limits paper consumption. Moreover, the aggregation of this data opens up innovative perspectives in the early detection of material losses, a major issue in the food industry.
Related News
